
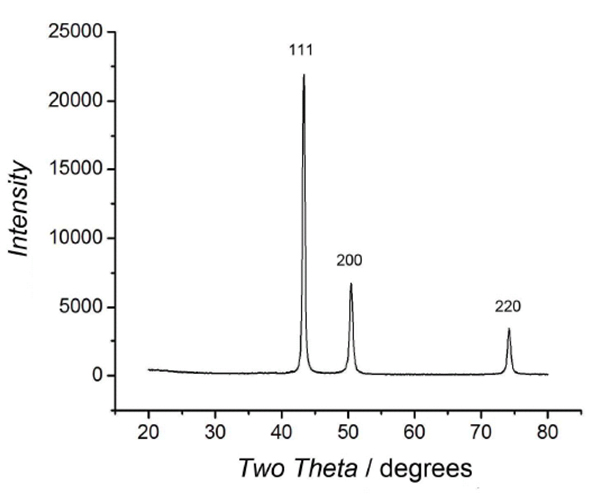
Preparation of Cu NPs/bentoniteįor green synthesis of Cu NPs/bentonite composite, 10 g natural bentonite was dispersed in 100 mL of 0.2 M CuSO 4♵H 2O under continuous stirring. This solution of the extract was used for the synthesis of Cu NPs/bentonite. Preparation of Thymus vulgaris extractĥ g of a dried powder of Thymus vulgaris leaves and pedicles was extracted by boiling in 30 mL double distilled water for 15 min and the aqueous extract was centrifuged at 7000 rpm to obtain the supernatant as an extract. The specific surface area was calculated from the BET equation. The pore distributions and pore volumes were calculated using the adsorption branch of the N 2 isotherms based on the Barrett–Joyner–Halenda (BJH) model. Nitrogen adsorption isotherms were performed on a volumetric gas adsorption apparatus (BEL Japan, Belsorp-max). TEM images were obtained using a Philips-EM-2085 transmission electron microscope with an accelerating voltage of 100 kV. The chemical composition of the modified bentonite was measured by EDX performed in a SEM. The morphology and particle dispersion was investigated by FE-SEM (Cam scan MV2300). The XRF analysis of the catalyst was performed with a Bruker S4 instrument. XRD analysis was performed on a Philips powder diffractometer type PW 1373 goniometer, which was equipped with a graphite monochromator crystal. Thin-layer chromatography (TLC) was performed on silica gel polygram SIL G/UV 254 plates. The melting points were taken in open capillary tubes with a Büchi 510 melting point apparatus and were uncorrected. The NMR spectra were obtained on a Brucker Avance 90 MHz spectrometer, using tetramethylsilane (TMS) as an internal standard. The IR spectra were recorded on a JASCO, FT/IR-6300 instrument in KBr pellets. The bentonite and Thymus vulgaris plant used in this paper were collected from the Vartoon region (Isfahan, Iran). Īll reagents were purchased from the Merck and Sigma-Aldrich and used without further purification. Thymol ( 1), carvacrol ( 2), p-cymene ( 3) and γ-terpinene ( 4) are the major components found in Thymus vulgaris ( Scheme 1). Therefore, the use of plants as a natural and biological source for biosynthesis of nanoparticles should be explored.Ĭommon thyme with the scientific name Thymus vulgaris is one of the plants indigenous to Iran which is valued for its antiseptic and antioxidant properties. The plant biosynthesis of nanoparticles immobilized on natural supports is a subject of new research as little has been published on this topic. During recent research on tetrazoles, 1 -substituted 1 H -1,2,3,4 -tetrazoles was found to be a special category due to their biological activity. Therefore, nowadays attention and progress in synthesis of these heterocycles play an essential role in organic, medicinal and synthetic chemistry. Tetrazoles are among the heterocycles most applied in medicine and industry due to their structural potential such as their usage as an isosteric substituent for carboxylic acids, analytical reagents and biological applications. These layered materials are very promising supports for the design and preparation of green catalysts. Smectites are major clay minerals in bentonite with an aluminum octahedral sheet sandwiched between two silica tetrahedral sheets. In recent decades, the use of natural bentonites has been studied due to their high specific surface area, low cost, ordered structure, thermal stability, high safety, high exchange capacity and intercalation abilities. However, most of these supports suffer from inefficiency to achieve highly distributed and stable metal NPs. The extremely small scale of nanoparticles (NPs) is the main factor leading to their surprising reactivity as compared to their corresponding bulk metals. Metal nanoparticles immobilized on supports such as carbon, zeolites, clay, metal oxides, graphene, etc., have been successfully applied as heterogeneous catalysts due to their interesting structures and properties. The development of new methodologies for the preparation of heterogeneous catalysts is of great interest in organic synthesis.


 0 kommentar(er)
0 kommentar(er)
